What is a polymer?
A polymer is essentially made up from small organic molecules linked up into long chains. In some cases the structures are linear, others have pendant groups that project on either side of the main chain (the monomer unit).
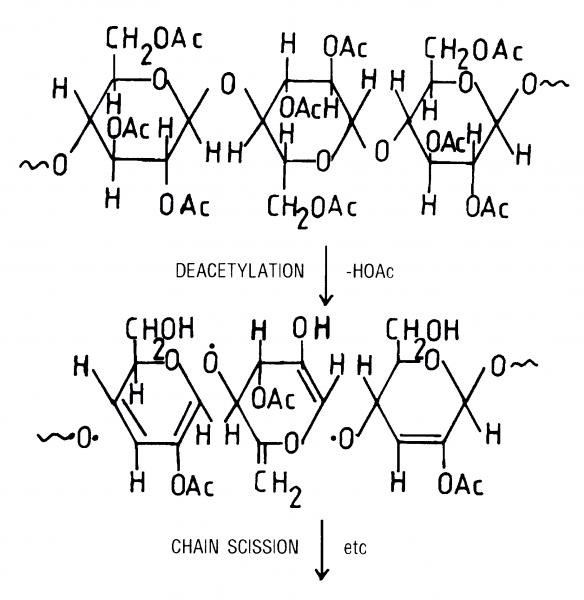
Figure 1
If we look at the chemical structure of a typical motion picture film support such as cellulose nitrate and cellulose triacetate (included in Figure 1), we see that basically it’s composed of a cellulose backbone made of small hydroglucose units linked together to form a long chain polymer; substituted in that main backbone are either nitrate groups (in the case of cellulose nitrate) or acetate groups (in the case of cellulose acetate and cellulose triacetate). So, our support material is a polymer which is a backbone with pendant groups.
A number of energy considerations have to be taken into account when looking at the stability of a particular structure. The first are the intramolecular forces - the bonds between each atom within the molecule. Basically, the stronger the chemical bond, the more stable the polymer will be and the more energy it takes to break that bond.
For example, a carbon-fluorine bond would be need 116kCal of energy per mole to break it; the carbon-hydrogen bond (present in cellulose acetate and cellulose nitrate) requires only 97kCal per mole; the carbon-carbon bond (also present in both support materials) requires only 83kCal per mole. The stability of the polymer also depends on the type of carbon-hydrogen bond present. If the carbon’s bonded to three hydrogen atoms, for example, it’s more stable than if the carbon’s doubly-bonded to another carbon: instead of just one bond, there are two.
If we look at intermolecular forces, we find that the many, many polymer chains within our plastic support will interact with each other and form weak links between the chains.
If you wrote a league table of bond strengths within polymer systems, then the bonds between the atoms that constitute the polymer chain take most energy to be broken, the intermolecular forces take a little less, and there’s essentially a cohesive energy between the chains. The cohesive energy in a rubber system, an elastomer which you can stretch, is pretty low compared to, say, a nylon or cellulose fibre where the cohesive forces will be much stronger. The presence of polar groups and hydrogen bonding provides the possibility of relatively strong links between each particular chain. So when you examine a polymer structure, you have to take into account whether all those forces or bond linkages can actually occur.


