What happens when support materials break down?
Using the particular examples of the support materials cellulose acetate and cellulose nitrate, how do we actually utilize information about the structure and structural stability to study what’s occurring when those two support materials breakdown?
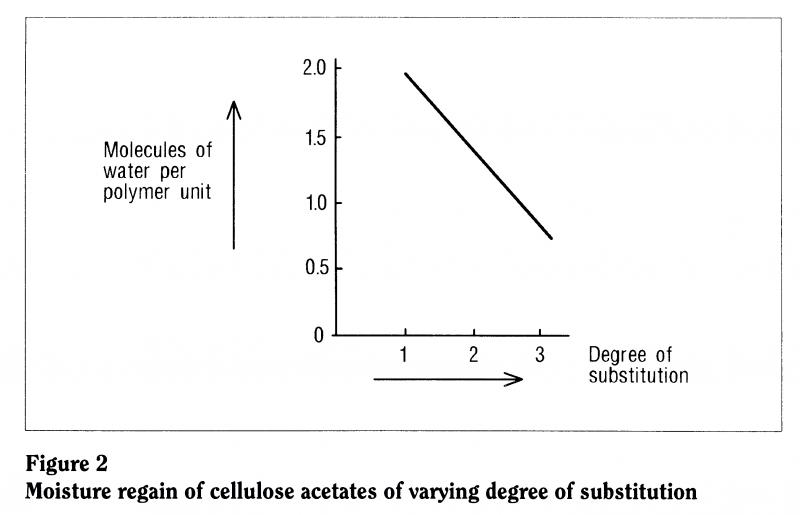
A particularly important feature of cellulose acetate and triacetate and, to a lesser degree, cellulose nitrate base is the extent to which the polymer can interact with the environment and take up moisture. Studies have shown that, as you increase the degree of substitution (as you go from monoacetate to diacetate to triacetate, see Figure 1), then you lower the moisture uptake of the polymer. The higher the degree of substitution, the less moisture it takes up. So conversely, if the polymer is degrading C giving off acetic acid, losing acetate groups, lowering the degree of substitution Cit will start to take up more moisture (Figure 2).
Another feature is the polymer’s solubility. The specific structural composition of the molecule will influence the way it interacts with solvents and hence will influence which solvent systems it can actually dissolve in. For example, at higher degrees of substitution, cellulose acetate is soluble in systems like methyline chloride and methanol which is actually used to cast film base and, as at lower degrees of substitution, it becomes increasingly soluble in more polar solvents until, at degrees of substitution of only 0.6 acetate groups per anhydroglucose monomer unit, the system should be soluble in water. You can actually utilize that information to study what is happening in your film material.
At Manchester Polytechnic, we took a series of films - naturally aged cellulose acetate films of unknown history, and a number of negative and positive films that we knew the dates of - and looked at them in several ways. Our initial observations of what happens to triacetate film when it degrades suggest that, when we smell acetic acid, this has come from acetate groups - the pendant groups that are substituted on the main chain. If we’re losing those groups, we’re lowering the degree of substitution and, therefore, we should see an increase in the uptake of moisture. We should also see, as we form acetic acid, a lowering in what we call the pH of the system. [If something has a high pH, say 13 or 14, it’s essentially alkaline/basic. If it has a very low pH, it’s tending towards being acidic.]
We can also utilize the information on solubility in a given solvent by measuring what we call percentage insoluble polymer. If we’re losing acetate groups (lowering the degree of substitution) then, if we could dissolve our original triacetate material in something like methyline chloride and methanol, it should become progressively insoluble in that particular solvent. So we can get a measure of percentage insoluble polymer.
We can also measure the viscosity of the system. We’re losing the pendant groups from our long chain to form acetic acid and the fact that we see a loss in tensile strength when we project films (the film snaps in the projector), suggests that the polymer unit itself, the main backbone, is also breaking down to smaller fragments. For a long chain, you can imagine that all the chains are entangled and it’s a relatively viscous solution. As the chain breaks into smaller fragments, it flows more freely. We should be able to follow that by measuring how viscous the solution is.
When we look through films taken from an archive, we find that some films have lost their image and also smell of acetic acid and that, the more degraded the film material, the higher percentage insoluble polymer it has, the lower its pH, the higher the moisture regain, and the lower the viscosity number. This seems consistent with the idea that the polymer is actually losing acetate groups to form acetic acid, and its main chain backbone is breaking down to smaller fragments.


