Polyvinyl chloride
A slightly different form of PVC to that used in substrates is favoured by European manufacturers as a binder, despite a slight tendency to shed oxide even when new. (Fig.1)
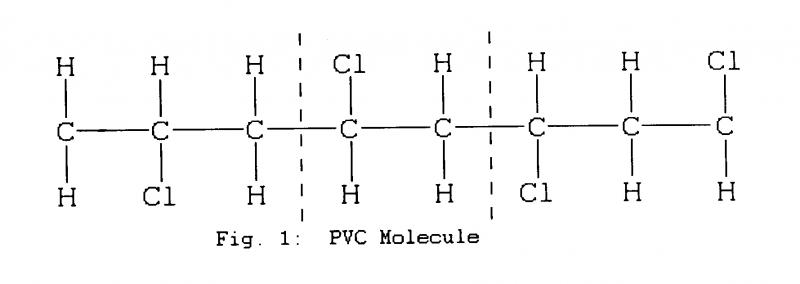
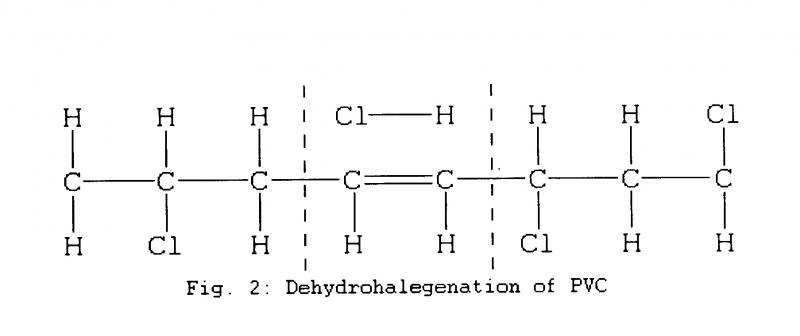
PVC typically breaks down by losing hydrogen and chlorine molecules which can produce hydrochloric acid (HC1) and leave double carbon bonds. (Fig.2)
Unlike the relatively rigid vinyl discs which incorporate a proportionally far smaller load of pigment as well as stabilisers to prevent decomposition, tapes typically incorporate 40% or more by volume of magnetic particles or pigment along with the binders themselves.


