Polyester-urethanes
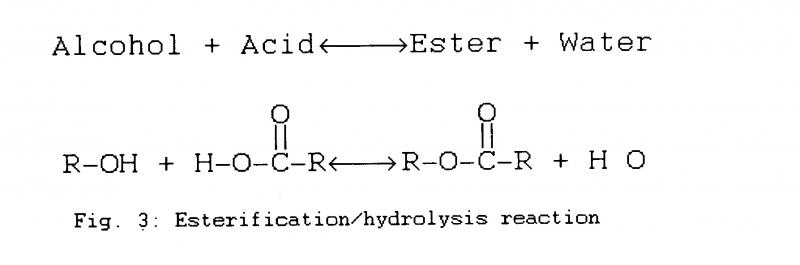
Polyesters are formed by reacting carboxylic acid and an alcohol to produce esters and water. This esterification reaction is reversible by a process of hydrolysis, in which water and esters are consumed, whilst acid and alcohol are produced (Fig.3). Being a reversible reaction, it is an equilibrium equation; in the presence of a certain moisture concentration, i.e. airborne humidity. polyesters will evolve or consume water from the surroundings until an equilibrium is reached.
The PE-U in tape binders are typically cross-linked co-polymers. The chains are arranged in lattice-shaped structures joined by ester linkages. These aliphatic esters which number roughly 5 or 10 times as many as the polyurethanes are much more prone to hydrolyse than the PET substrate 5.


