Photographic Film
Table 1 illustrates the characteristics of the breakdown of photographic film materials as a function of the plastic base which supports the emulsion. The plastic is made up of a polymer mixed with various additives.
| Table 1 | |
|---|---|
| Nitrate Film | Acetate Film |
| Acrid colours | Vinegar odour |
| Film discolours | Warping |
| Image fades | Shrinkage |
| Sticky emulsion | White crystals on film surface |
| Film yellows and congeals | Film reel flaccid or crystalline |
| Large volumes of brown powder on film or in can |
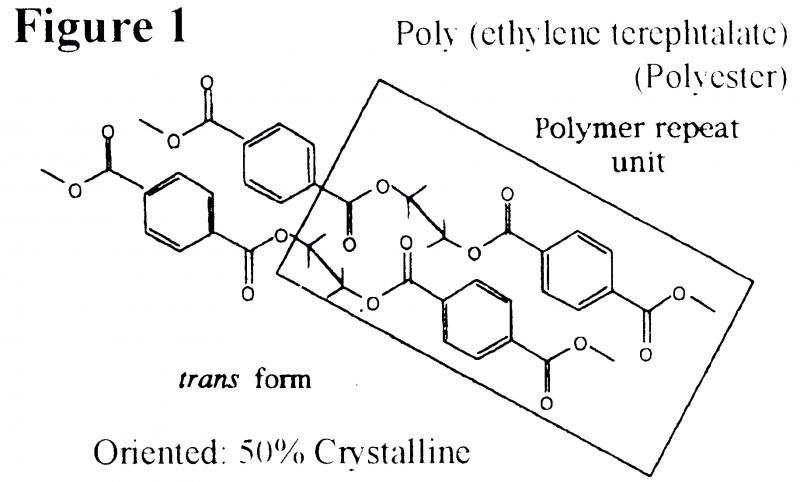 Polymers are sensitive to their environment and degrade by both hydrolysis and oxidation: They consist of small structural units which are linked together and continuously repeated to form chains. This is illustrated in Figure 1, which shows the chemical structure of two polyester chains, lying side-by-side with the repeat unit within each chain highlighted.
Polymers are sensitive to their environment and degrade by both hydrolysis and oxidation: They consist of small structural units which are linked together and continuously repeated to form chains. This is illustrated in Figure 1, which shows the chemical structure of two polyester chains, lying side-by-side with the repeat unit within each chain highlighted.
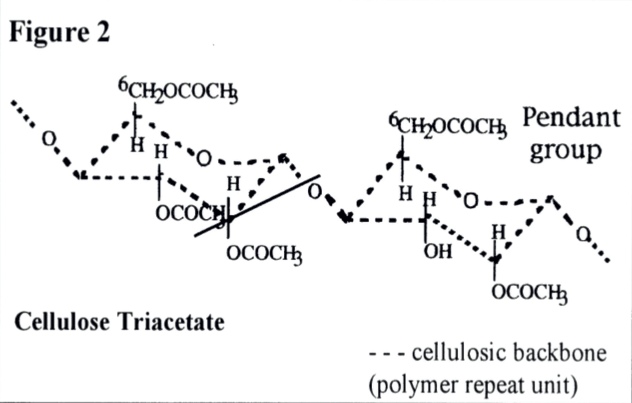 In some polymers pendent groups are present along the chain at regular intervals (e.g. as in cellulose nitrate and cellulose acetate (Figure 2)). Both the link points in the chains and the pendant groups are sensitive to moisture, heat and light, hence their environment facilitates hydrolysis and oxdation.
In some polymers pendent groups are present along the chain at regular intervals (e.g. as in cellulose nitrate and cellulose acetate (Figure 2)). Both the link points in the chains and the pendant groups are sensitive to moisture, heat and light, hence their environment facilitates hydrolysis and oxdation.
Where pendant groups are present on the polymer chain they may be released: For example, in the presence of moisture the pendant ester group of cellulose esters is released to form an acid (in cellulose nitrate this is nitric acid and in cellulose acetate this is acetic acid. When the pendant group is released it diffuses through the film reel and out into the film can accounting for the acrid (nitric acid) and vinegar (acetic acid) odours that are all too frequently detected. As the acids diffuse through the film they also come into contact with the film emulsion (silver image + gelatin). Nitrogen dioxide and nitric acid are highly oxidising and fade the silver image to characteristic sepia tones.
Both acids (nitric and acetic) may decompose gelatin causing it to become sticky (nitric acid more so than acetic). In addition, in sufficient quantities these acids may instigate secondary reactions, breaking the links in the polymer chains leading to a reduction in tensile properties and dimensional stability of the film. Since the acid which is initially formed facilitates further breakdown, the degradation reaction is said to be auto catalytic.


