The effect of the film can
But that’s still not the whole story. The film is not just sitting on the shelf tightly wound into a reel, it’s actually in a film can. Is the can itself contributing to the instability of the material?
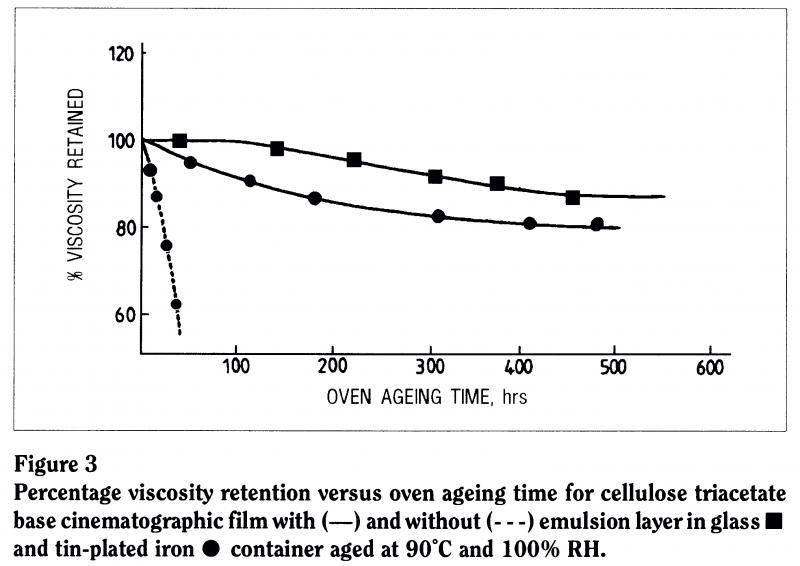
Metal ions are notorious in the breakdown of polymers. The chemical literature tells us that metal ions contribute to the instability of almost every known polymer system: they act as catalysts for their breakdown. We looked at what happens to photographic film, motion picture film and triacetate base in contact with a can. In one case, we removed the emulsion from the film, in another we left it on, and in a third case we left the emulsion on and aged the film in contact with glass rather than with a metal storage can (Figure 3). The support without emulsion tends to degrade first, the film with emulsion in contact with the can takes a little longer, but the film in a glass container is much more stable.
We also looked at a number of naturally aged film materials taken from film archives, at new materials, at film that was exhibiting acetic acid odour, at films that had plasticizer deposits, and at films that had lost the image altogether. The analysis was done by Kodak in England who found that, the more degraded the film was, the greater its iron content. In other words, the film material, as it degrades, seems to be taking up iron. Now it’s difficult to tell from those two studies whether or not this is a facile feature of the degradation. It maybe that, because the film takes up more moisture it also takes up more metal ions along with that; so the iron isn’t actually contributing to the degradation, it’s just being taken up as the film degrades.
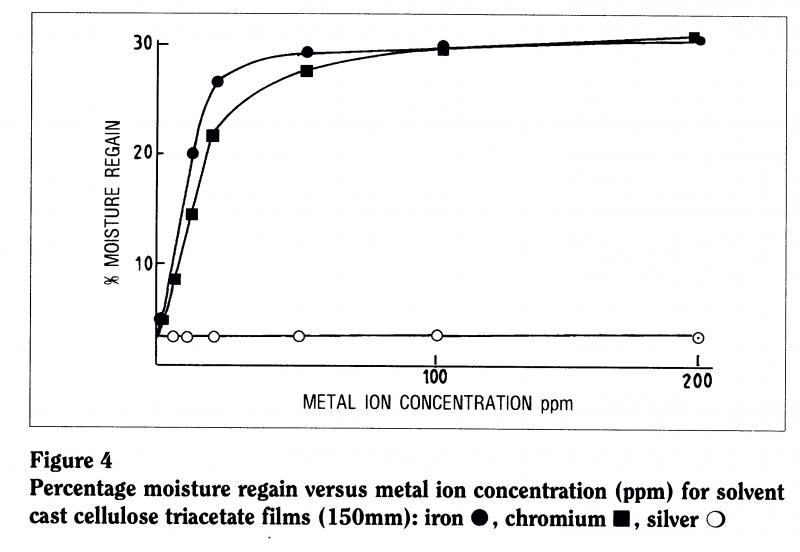
To check that, we actively incorporated different concentrations of metal ions within a number of different films (Figure 4). When we put one part per million of ions into the material, there wasn’t much change in the viscosity the film wasn’t degrading very much. But, as we increased the concentration to ten parts per million, there was significant loss of viscosity Csignificant degradation. In other words, iron contributes to the instability of the material. So it’s likely that the film can is contributing to the instability of the material.
And with magnetic track media, because the magnetic track is an iron oxide or iron itself, then that must also be contributing to the instability. We took from the archives a cross section of materials that we knew were exhibiting degradation and we looked at the percentages of degraded film materials exhibiting acetic acid odour (or vinegar syndrome). We found that 78% of the degraded materials were magnetic track materials, compared with only 22% that had no magnetic track and so had no iron present in the base material.
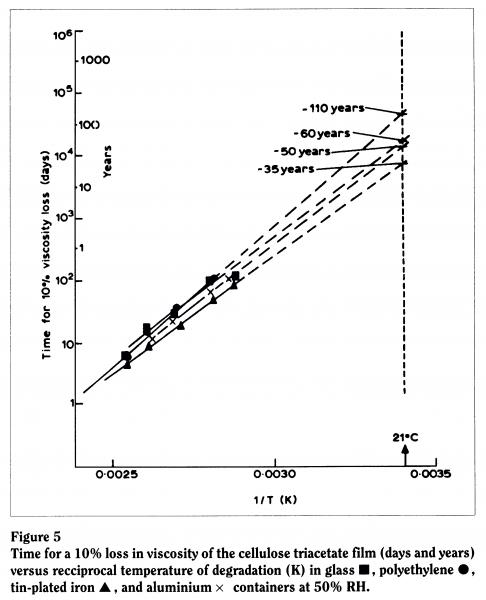
Because we realized that the effect of the film can was particularly important, we used Arrhenius testing methods to ascertain the time taken for a given loss in a physical property of the material. For example, we plotted the time taken for a 10% loss in viscosity against temperature in various storage media. At 50% relative humidity (the humidity level found in many archival storage facilities), we found that film was most stable in glass, then plastic, then aluminium, and least stable in contact with a tin-plated can. We predicted that the lifetime of the material in contact with a typical tin-plated can in an archive would be around 35 years (Figure 5). Allowing for experimental error and any error in our plotting (given that we’re plotting against a log scale, our line could be out by "10 years), this corresponds reasonably well with what we actually find because, if you recall, triacetate was introduced in the early fifties and we’re seeing degradation now.