How do the polymers degrade?
Photographic Film
Table 1 illustrates the characteristics of the breakdown of photographic film materials as a function of the plastic base which supports the emulsion. The plastic is made up of a polymer mixed with various additives.
| Table 1 | |
|---|---|
| Nitrate Film | Acetate Film |
| Acrid colours | Vinegar odour |
| Film discolours | Warping |
| Image fades | Shrinkage |
| Sticky emulsion | White crystals on film surface |
| Film yellows and congeals | Film reel flaccid or crystalline |
| Large volumes of brown powder on film or in can |
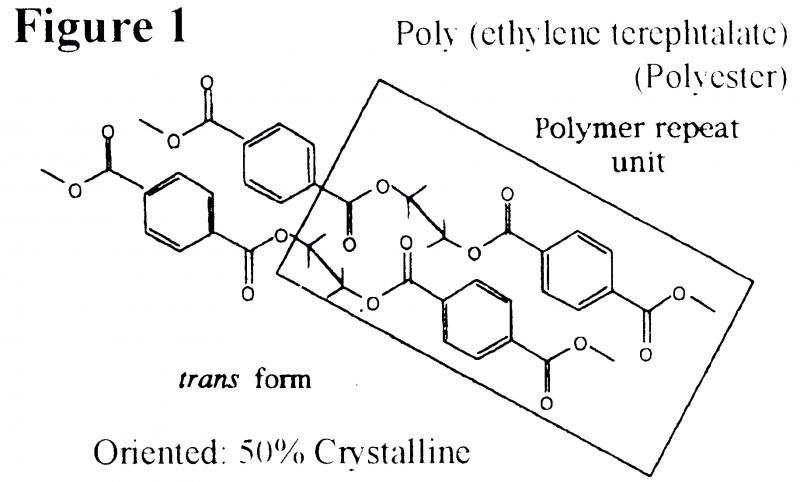 Polymers are sensitive to their environment and degrade by both hydrolysis and oxidation: They consist of small structural units which are linked together and continuously repeated to form chains. This is illustrated in Figure 1, which shows the chemical structure of two polyester chains, lying side-by-side with the repeat unit within each chain highlighted.
Polymers are sensitive to their environment and degrade by both hydrolysis and oxidation: They consist of small structural units which are linked together and continuously repeated to form chains. This is illustrated in Figure 1, which shows the chemical structure of two polyester chains, lying side-by-side with the repeat unit within each chain highlighted.
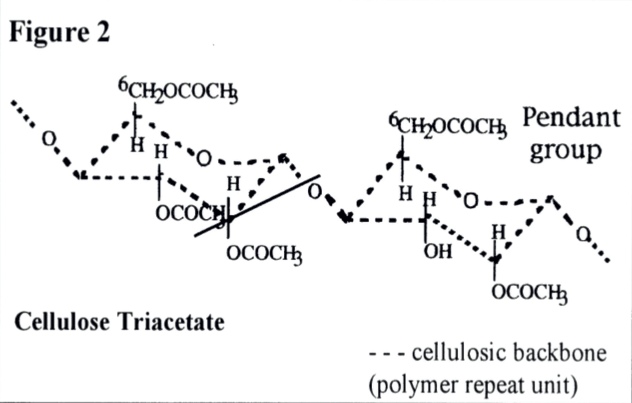 In some polymers pendent groups are present along the chain at regular intervals (e.g. as in cellulose nitrate and cellulose acetate (Figure 2)). Both the link points in the chains and the pendant groups are sensitive to moisture, heat and light, hence their environment facilitates hydrolysis and oxdation.
In some polymers pendent groups are present along the chain at regular intervals (e.g. as in cellulose nitrate and cellulose acetate (Figure 2)). Both the link points in the chains and the pendant groups are sensitive to moisture, heat and light, hence their environment facilitates hydrolysis and oxdation.
Where pendant groups are present on the polymer chain they may be released: For example, in the presence of moisture the pendant ester group of cellulose esters is released to form an acid (in cellulose nitrate this is nitric acid and in cellulose acetate this is acetic acid. When the pendant group is released it diffuses through the film reel and out into the film can accounting for the acrid (nitric acid) and vinegar (acetic acid) odours that are all too frequently detected. As the acids diffuse through the film they also come into contact with the film emulsion (silver image + gelatin). Nitrogen dioxide and nitric acid are highly oxidising and fade the silver image to characteristic sepia tones.
Both acids (nitric and acetic) may decompose gelatin causing it to become sticky (nitric acid more so than acetic). In addition, in sufficient quantities these acids may instigate secondary reactions, breaking the links in the polymer chains leading to a reduction in tensile properties and dimensional stability of the film. Since the acid which is initially formed facilitates further breakdown, the degradation reaction is said to be auto catalytic.
Magnetic Tape
It is inopportune that more recent audio-visual media are also exhibiting breakdown, though perhaps we should not be surprised since they too are composed of polymers.
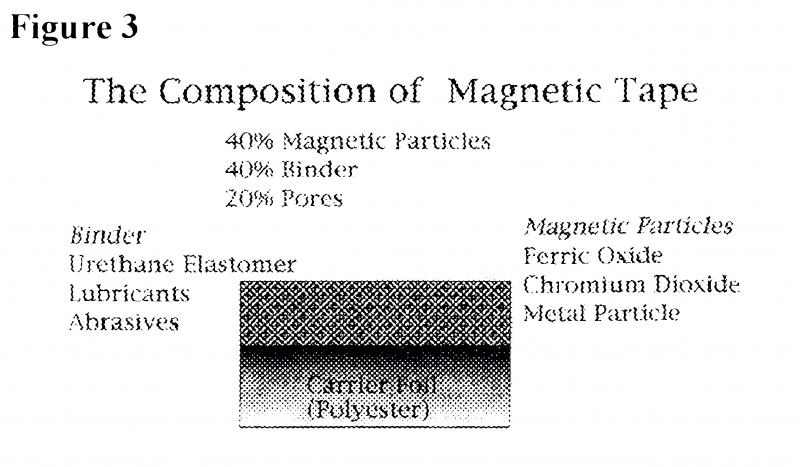 Figure 3 illustrates the composition of magnetic tape. This medium consists of 40% (by volume) of magnetic particles (yFe2O3, Cr02 or metal particles), 40% polymeric binder (urethane or PVC elastomer, plus lubricants and abrasives) and 20% pores (to reduce friction arising from contact with tape heads on playback) which together constitute the information carrying layer. This layer is the one responsible for instability of the tape, the polyester carrier foil onto which it is coated is considered stable. The characteristics of chemically degraded magnetic tapes include the presence of sticky or particulate deposits on the tape surface and/or binder shedding.
Figure 3 illustrates the composition of magnetic tape. This medium consists of 40% (by volume) of magnetic particles (yFe2O3, Cr02 or metal particles), 40% polymeric binder (urethane or PVC elastomer, plus lubricants and abrasives) and 20% pores (to reduce friction arising from contact with tape heads on playback) which together constitute the information carrying layer. This layer is the one responsible for instability of the tape, the polyester carrier foil onto which it is coated is considered stable. The characteristics of chemically degraded magnetic tapes include the presence of sticky or particulate deposits on the tape surface and/or binder shedding.
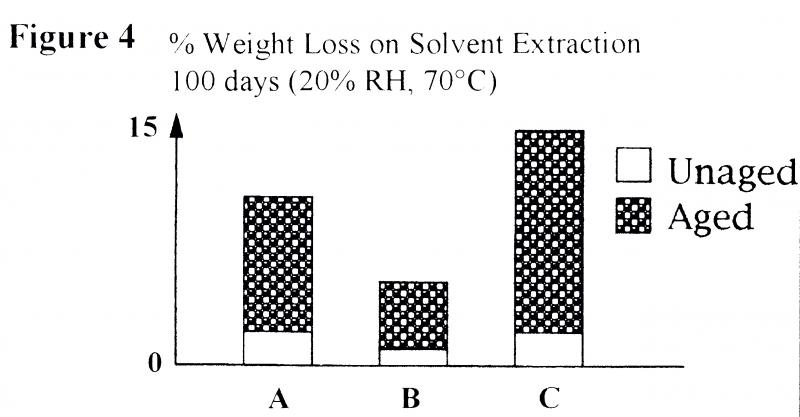 Figure 4 illustrates the weight loss experienced on solvent extraction of three commercial magnetic tapes. The weight loss in unaged tapes is due to extraction of additives, such as lubricants from the binder layer.
Figure 4 illustrates the weight loss experienced on solvent extraction of three commercial magnetic tapes. The weight loss in unaged tapes is due to extraction of additives, such as lubricants from the binder layer.
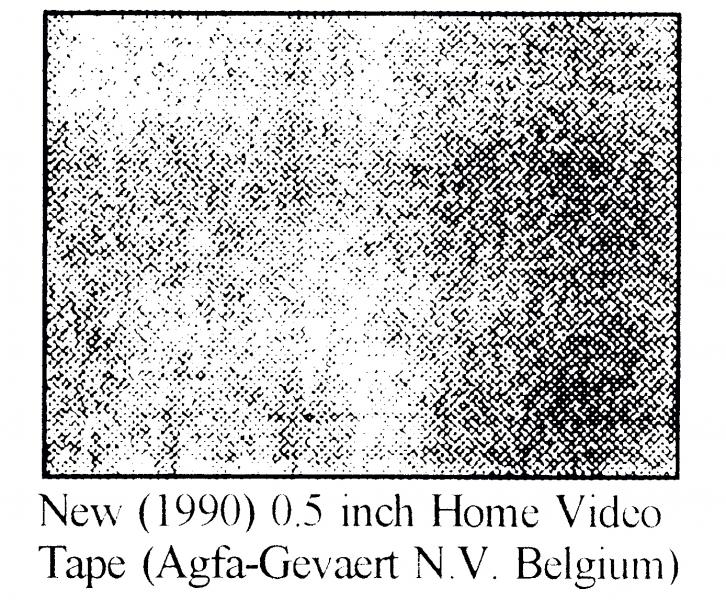
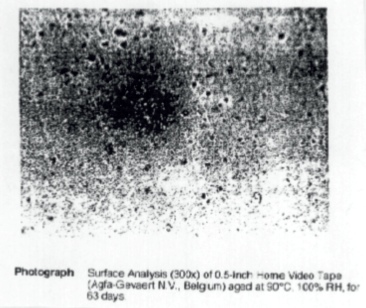
Figure 5 shows photographs taken when magnetic tapes were examined at high resolution using a Vanox microscope. Lubricants which have migrated to the film surface and then deposited can be clearly seen in older and aged magnetic tapes. A much greater weight loss is observed (Figure 4) when tapes are aged at 20% RH and 70ºC for 100 days. This results in oxidation and hydrolysis of the binder, and it should be noted that the quantity of extractable materials resulting from breakdown varies from tape to tape.
 |
 |
 |
 |
Figure 5 Surface analysis photographs of tapes taken by a Vanox microscope at 300X (a) 0.5 inch Home Video Tape (Agfa-Gevaert N.V. Belgium) (b) 0.5 inch Home Video Tape (Agfa-Gevaert N.V. Belgium) aged at 90°C, 100% RH for 63 days. (c) 2 inch Commercial Video Tape (Scotch) made in 1976 (NFTVA, England) (d) 2 inch Commercial Video Tape (Scotch) made in 1968 (NFTVA, England)
Studies clearly indicate that for urethane elastomers oxidation precedes hydrolysis. Attempts to recover information from degraded magnetic tapes and an examination of signal quality clearly demonstrates that breakdown is not simply hydrolysis but must involve other processes (refer to the work of Kevin Bradley).
Table 2 shows the results of an examination of the oxidative breakdown of the three commercial tapes. Two parameters are tabulated: initial peroxide concentration and carbonyl index (time to 0.1 C-O). Peroxides are formed in the binder during and subsequent to manufacture of the tapes.
These species may decompose and instigate the degradation of the binder, It can be seen that initial peroxide levels are significantly different since they depend upon the specific manufacturer formulation. However, this cannot in itself be used as a guide to tape stability because peroxide stability is determined by binder structure and the types of metal ions present (oxidation is in this case exacerbated by the presence and nature of the metal particles embedded in the binder). Breakdown of peroxides results, ultimately, in the production of carbonyl compounds within the binder and measurement of the rate of production of these species correlates to binder shedding.
| Table 2 | ||
|---|---|---|
| Manufacturer | Initial Peroxide Concentration (mg/g) |
Time to 0.1 C-O (days)* |
| A | 19 | 170 |
| B | 5.6 | 210 |
| C | 2.2 | 140 |
* 20% RH, 70ºC
Table 2 indicates that, though Tape C has the lowest initial peroxide level, it takes the shortest time to develop 0.1 carbonyl. After 140 days the magnetic oxide was easily brushed away from the tape surface. It should be evident by now that significant progress has been made in defining the nature of decomposition in audio-visual materials, but the key question remains
"How can the lifetime of such materials be extended?”