Binders
Of the 3 main components in modern formulations, the binder system is considered to be the weakest. IASA's own literature over the last two decades has addressed a wide range of issues from print-through, stray magnetic fields and even detailed arguments about period playback equipment, without ever having focused on binders. Could it be that this is a purely antipodean issue? Recent industry articles in both the U.S.A. and the U.K. seem to suggest otherwise 3,4.
The binder probably has the toughest job of the three primary components. It has to perform several roles simultaneously:
Cohesion - it must hold the magnetic particles or back-coat together in a tough, wear-resistant layer of uniform dispersion, with each particle thoroughly coated, but without itself occupying excessive volume so as to reduce the density and therefore, signal carrying capacity of the tape;
Flexibility - to withstand successive bending and straightening from spooling/ unspooling and curving around the heads and guides of the tape transport.
Runability - it must allow the passage of tape through the transport with minimum friction and mechanical interference - flapping around, “stiction” or other irregularities which can cause flutter, modulation noise and related problems.
The two main families of binders in current use are polyester-urethanes (PE-U) and (PVC). Examples of PVC include drainpipes, shoe soles and LP records, which are believed to able to last for a century or more. PE-Us are used in such demanding applications as motor vehicle suspension bushes, casting or moulding of precision mechanical and electrical components, skateboard/roller-blade tyres and some of the toughest, most chemical resistant lacquers and finishes ever invented. Whilst neither is clearly superior, their success as tape binders depends on adherence to strict manufacturing tolerances, and on the exact composition, which can incorporate a host of additional ingredients such as lubricants, plasticisers, stabilisers, fungal inhibitors, anti-foaming agents, dispersants and the like.
Polyvinyl chloride
A slightly different form of PVC to that used in substrates is favoured by European manufacturers as a binder, despite a slight tendency to shed oxide even when new. (Fig.1)
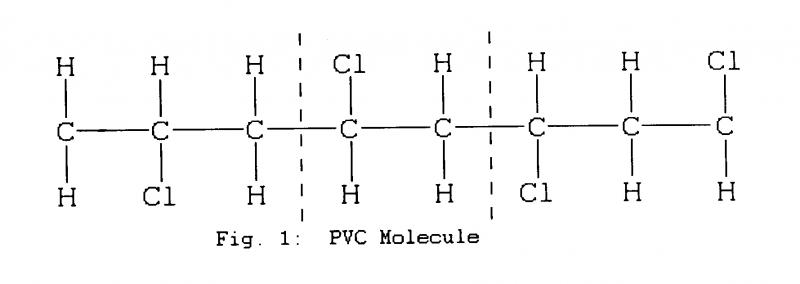
PVC typically breaks down by losing hydrogen and chlorine molecules which can produce hydrochloric acid (HC1) and leave double carbon bonds. (Fig.2)
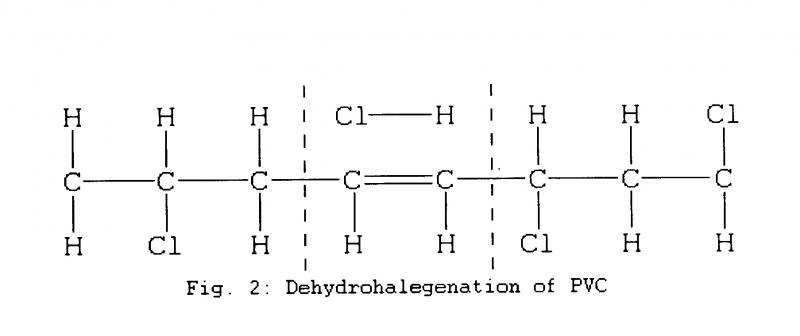
Unlike the relatively rigid vinyl discs which incorporate a proportionally far smaller load of pigment as well as stabilisers to prevent decomposition, tapes typically incorporate 40% or more by volume of magnetic particles or pigment along with the binders themselves.
Polyester-urethanes
Polyesters are formed by reacting carboxylic acid and an alcohol to produce esters and water. This esterification reaction is reversible by a process of hydrolysis, in which water and esters are consumed, whilst acid and alcohol are produced (Fig.3). Being a reversible reaction, it is an equilibrium equation; in the presence of a certain moisture concentration, i.e. airborne humidity. polyesters will evolve or consume water from the surroundings until an equilibrium is reached.
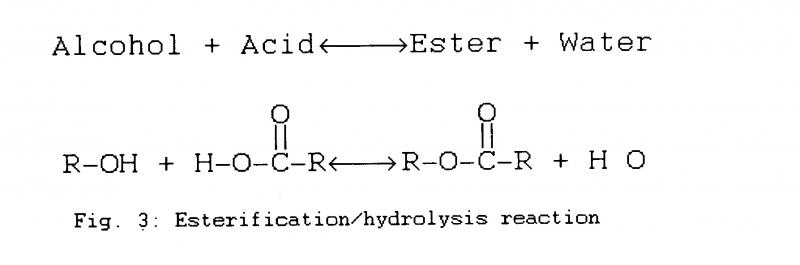
The PE-U in tape binders are typically cross-linked co-polymers. The chains are arranged in lattice-shaped structures joined by ester linkages. These aliphatic esters which number roughly 5 or 10 times as many as the polyurethanes are much more prone to hydrolyse than the PET substrate 5.