The decay of polymers in information storage carriers
THE DECAY OF POLYMERS IN INFORMATION STORAGE CARRIERS
Dr Michele Edge and Joan Whitehead – presented at 1995 JTS in London; printed in the Proceedings pages 19 – 30.
No ISBN
IASA members may download the PDF version at no cost. If you are not a member, why not join IASA?
Published by the Technical Co-ordinating Committee.
MICHELE EDGE
assisted by
Joan Whitehead,
Centre for Archival Polymeric Materials,
Manchester Metropolitan University.
Introduction by Henning Schou:
Dr Edge gained her Degree and PhD from Manchester Polytechnic (now Manchester Metropolitan University). She is currently Lecturer in Physical Chemistry and is also involved in the supervision of 12 PhDs in Applied Polymer Chemistry, Polymer Photochemistry and Conservation of Polymers. The work of the researchers is sponsored by the National Film and Television Archive, the Tate Gallery, the Science Museum, ICI, Courtaulds Coatings, Du-Pont, Tioxide and Unilever.
Dr Edge is the Co-Director of the Centre for Archival Polymers which, with her colleagues at the University, Professor Norman Allan and Dr. Jewitt, and Velsom Horrie of Manchester Museum, she founded at Manchester Metropolitan University (formerly Manchester Polytechnic). The Centre undertakes research and consultancies for polymers used in long-term applications including information carriers. She is a member of the committee of the Makro group of the Royal Society of Chemistry for discussion of polymer degradation.
Those of you who were in Ottawa in 1990 will recall Dr Edge ‘s presentation which dealt with the deterioration of polymers in audiovisual materials. Today, five years on from Ottawa, Dr. Edge will summarise the progress made in our understanding of the stability of information carriers. She will be assisted with the presentation by her PhD student, Joan Whitehead.
Table of contents
Summary of Paper
This paper provides a summary of the progress made to date in our understanding of the stability of information carriers. Each of the following problems will be addressed:
- How do the polymers that make up information carriers breakdown. What is special about the structure of the materials used as information carriers which make them susceptible to the environment.
- How to identify breakdown. What is the final point at which a material that is degrading but the information can be recovered. At what point does the breakdown become irretrievable. What happens in the “grey area” between these two points. Here simple observation of materials, together with an evaluation of “chemical” test methods, will be covered. Emphasis will be placed on the value of identifying which materials are at risk of deterioration.
- How is breakdown influenced by archival storage conditions. Temperature, humidity and storage containers will be discussed.
- How can the life expectancy of such materials be extended. Information carrier materials do not last forever but we can double, or even triple, their life by careful control of environmental conditions.
Introduction
This paper provides a summary of the progress made to date in our understanding of the stability of information carriers. It is not comprehensive but provides “snapshots” of key issues. Accordingly it addresses problems such as how information carriers break down, how to identify breakdown, how breakdown is influenced by archival storage and how we can extend the lifetime of carriers. With these questions in mind
- - - - - How do the polymers which make up information carriers degrade?
How do the polymers degrade?
Photographic Film
Table 1 illustrates the characteristics of the breakdown of photographic film materials as a function of the plastic base which supports the emulsion. The plastic is made up of a polymer mixed with various additives.
| Table 1 | |
|---|---|
| Nitrate Film | Acetate Film |
| Acrid colours | Vinegar odour |
| Film discolours | Warping |
| Image fades | Shrinkage |
| Sticky emulsion | White crystals on film surface |
| Film yellows and congeals | Film reel flaccid or crystalline |
| Large volumes of brown powder on film or in can |
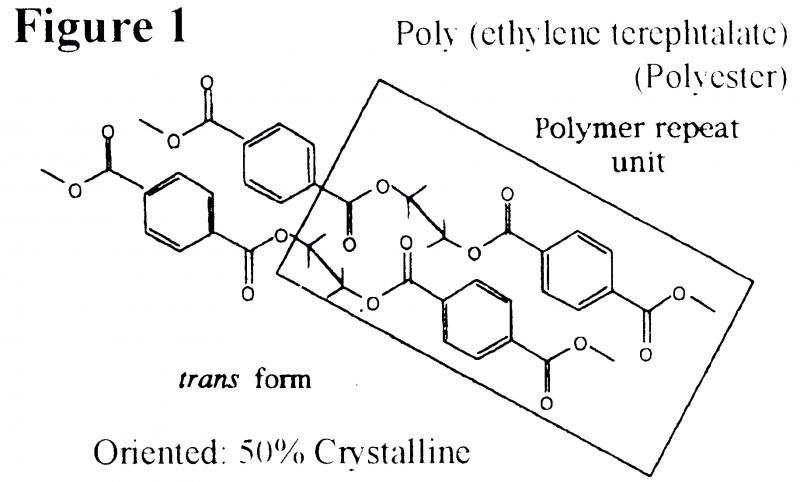 Polymers are sensitive to their environment and degrade by both hydrolysis and oxidation: They consist of small structural units which are linked together and continuously repeated to form chains. This is illustrated in Figure 1, which shows the chemical structure of two polyester chains, lying side-by-side with the repeat unit within each chain highlighted.
Polymers are sensitive to their environment and degrade by both hydrolysis and oxidation: They consist of small structural units which are linked together and continuously repeated to form chains. This is illustrated in Figure 1, which shows the chemical structure of two polyester chains, lying side-by-side with the repeat unit within each chain highlighted.
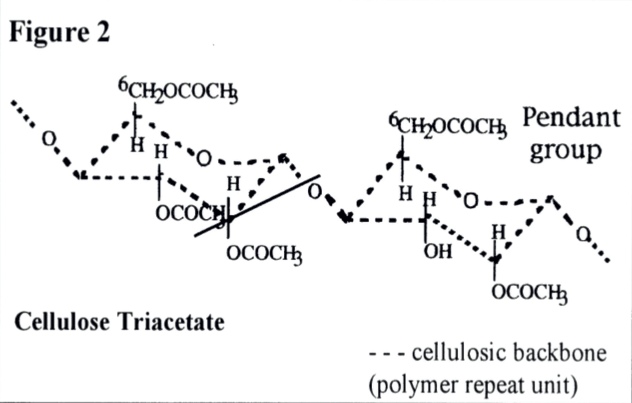 In some polymers pendent groups are present along the chain at regular intervals (e.g. as in cellulose nitrate and cellulose acetate (Figure 2)). Both the link points in the chains and the pendant groups are sensitive to moisture, heat and light, hence their environment facilitates hydrolysis and oxdation.
In some polymers pendent groups are present along the chain at regular intervals (e.g. as in cellulose nitrate and cellulose acetate (Figure 2)). Both the link points in the chains and the pendant groups are sensitive to moisture, heat and light, hence their environment facilitates hydrolysis and oxdation.
Where pendant groups are present on the polymer chain they may be released: For example, in the presence of moisture the pendant ester group of cellulose esters is released to form an acid (in cellulose nitrate this is nitric acid and in cellulose acetate this is acetic acid. When the pendant group is released it diffuses through the film reel and out into the film can accounting for the acrid (nitric acid) and vinegar (acetic acid) odours that are all too frequently detected. As the acids diffuse through the film they also come into contact with the film emulsion (silver image + gelatin). Nitrogen dioxide and nitric acid are highly oxidising and fade the silver image to characteristic sepia tones.
Both acids (nitric and acetic) may decompose gelatin causing it to become sticky (nitric acid more so than acetic). In addition, in sufficient quantities these acids may instigate secondary reactions, breaking the links in the polymer chains leading to a reduction in tensile properties and dimensional stability of the film. Since the acid which is initially formed facilitates further breakdown, the degradation reaction is said to be auto catalytic.
Magnetic Tape
It is inopportune that more recent audio-visual media are also exhibiting breakdown, though perhaps we should not be surprised since they too are composed of polymers.
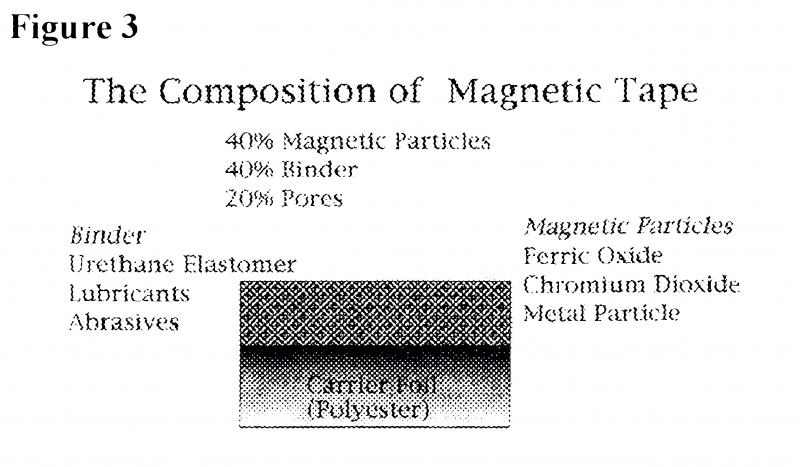 Figure 3 illustrates the composition of magnetic tape. This medium consists of 40% (by volume) of magnetic particles (yFe2O3, Cr02 or metal particles), 40% polymeric binder (urethane or PVC elastomer, plus lubricants and abrasives) and 20% pores (to reduce friction arising from contact with tape heads on playback) which together constitute the information carrying layer. This layer is the one responsible for instability of the tape, the polyester carrier foil onto which it is coated is considered stable. The characteristics of chemically degraded magnetic tapes include the presence of sticky or particulate deposits on the tape surface and/or binder shedding.
Figure 3 illustrates the composition of magnetic tape. This medium consists of 40% (by volume) of magnetic particles (yFe2O3, Cr02 or metal particles), 40% polymeric binder (urethane or PVC elastomer, plus lubricants and abrasives) and 20% pores (to reduce friction arising from contact with tape heads on playback) which together constitute the information carrying layer. This layer is the one responsible for instability of the tape, the polyester carrier foil onto which it is coated is considered stable. The characteristics of chemically degraded magnetic tapes include the presence of sticky or particulate deposits on the tape surface and/or binder shedding.
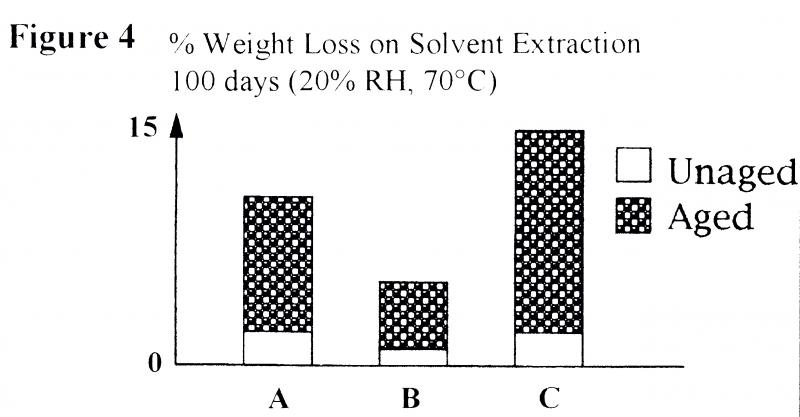 Figure 4 illustrates the weight loss experienced on solvent extraction of three commercial magnetic tapes. The weight loss in unaged tapes is due to extraction of additives, such as lubricants from the binder layer.
Figure 4 illustrates the weight loss experienced on solvent extraction of three commercial magnetic tapes. The weight loss in unaged tapes is due to extraction of additives, such as lubricants from the binder layer.
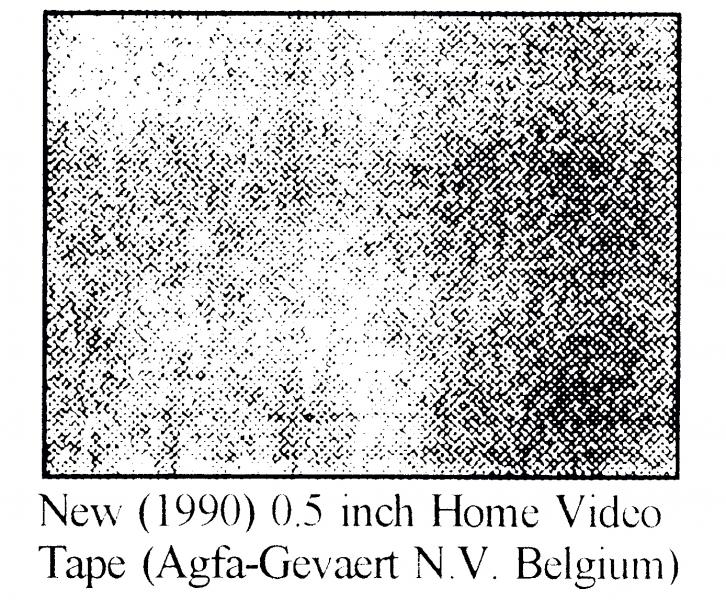
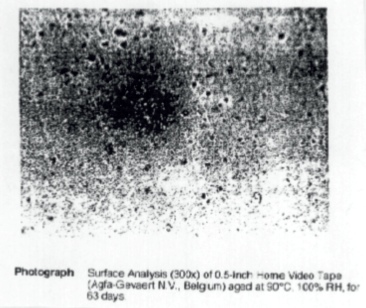
Figure 5 shows photographs taken when magnetic tapes were examined at high resolution using a Vanox microscope. Lubricants which have migrated to the film surface and then deposited can be clearly seen in older and aged magnetic tapes. A much greater weight loss is observed (Figure 4) when tapes are aged at 20% RH and 70ºC for 100 days. This results in oxidation and hydrolysis of the binder, and it should be noted that the quantity of extractable materials resulting from breakdown varies from tape to tape.
 |
 |
 |
 |
Figure 5 Surface analysis photographs of tapes taken by a Vanox microscope at 300X (a) 0.5 inch Home Video Tape (Agfa-Gevaert N.V. Belgium) (b) 0.5 inch Home Video Tape (Agfa-Gevaert N.V. Belgium) aged at 90°C, 100% RH for 63 days. (c) 2 inch Commercial Video Tape (Scotch) made in 1976 (NFTVA, England) (d) 2 inch Commercial Video Tape (Scotch) made in 1968 (NFTVA, England)
Studies clearly indicate that for urethane elastomers oxidation precedes hydrolysis. Attempts to recover information from degraded magnetic tapes and an examination of signal quality clearly demonstrates that breakdown is not simply hydrolysis but must involve other processes (refer to the work of Kevin Bradley).
Table 2 shows the results of an examination of the oxidative breakdown of the three commercial tapes. Two parameters are tabulated: initial peroxide concentration and carbonyl index (time to 0.1 C-O). Peroxides are formed in the binder during and subsequent to manufacture of the tapes.
These species may decompose and instigate the degradation of the binder, It can be seen that initial peroxide levels are significantly different since they depend upon the specific manufacturer formulation. However, this cannot in itself be used as a guide to tape stability because peroxide stability is determined by binder structure and the types of metal ions present (oxidation is in this case exacerbated by the presence and nature of the metal particles embedded in the binder). Breakdown of peroxides results, ultimately, in the production of carbonyl compounds within the binder and measurement of the rate of production of these species correlates to binder shedding.
| Table 2 | ||
|---|---|---|
| Manufacturer | Initial Peroxide Concentration (mg/g) |
Time to 0.1 C-O (days)* |
| A | 19 | 170 |
| B | 5.6 | 210 |
| C | 2.2 | 140 |
* 20% RH, 70ºC
Table 2 indicates that, though Tape C has the lowest initial peroxide level, it takes the shortest time to develop 0.1 carbonyl. After 140 days the magnetic oxide was easily brushed away from the tape surface. It should be evident by now that significant progress has been made in defining the nature of decomposition in audio-visual materials, but the key question remains
"How can the lifetime of such materials be extended?”
How can their lifetime be extended?
Control of environment is viewed as the primary means of extending the life of audio-visual materials. Because hydrolysis is the principal route to deterioration of a photographic film it is extremely difficult to inhibit by chemical means within the film matrix i.e. inhibition of moisture uptake by films has already been optimised by manufacturers. Since moisture is the species which brings about the hydrolysis reaction a dry atmosphere will prevent degradation.
Unfortunately, the gelatin of the film emulsion needs some moisture to prevent its embrittlement. When sufficient moisture is present, an increase in temperature will serve to accelerate the hydrolysis reaction. At higher temperatures, other reactions (free-radical processes) are facilitated which are normally insignificant at ambient temperatures.
Because of this latter feature, care must be exercised when carrying out accelerated ageing tests as a means to predict film lifetime: if the mechanism of degradation changes with increasing temperature then the relationship between time to a given property change and temperature will not be a linear one. Fortunately current standards relating to film storage have been derived from information compiled from accelerated ageing tests and from direct archival experience. This having been said the standards are still subject to debate, probably because they are a compromise between good intention, economics and what is attainable practically.
 A more ingenious way to use the available information pertaining to storage has been provided by the Image Permanence Institute's (IPI) Archival Storage Guides. Data taken from IPI*s Archival Storage Wheel is given in Table 3.
A more ingenious way to use the available information pertaining to storage has been provided by the Image Permanence Institute's (IPI) Archival Storage Guides. Data taken from IPI*s Archival Storage Wheel is given in Table 3.
The data give an indication of the relative lifetime of either a new film (or a degrading one) when kept under given conditions of temperature and relative humidity. information on the effect of time out of storage on film lifetime is also provided by the guides. Even if storage conditions recommended by standards are not within reach, this valuable data provides the archivist with an idea of the limits imposed on film life by the actual conditions implemented.
Standards relating to the keeping of magnetic materials are not as clearly laid down as those for photographic film, ostensibly due to the poorer understanding of relevant factors associated with their breakdown. In the case of magnetic materials because oxidation precedes hydrolysis there are several ways in which breakdown could be inhibited, and current research is concentrating on these.
We have already said that degradation is exacerbated by confinement of the acids evolved. Though the auto-catalytic reaction can be prevented by removing acid which is initially formed, it is not sufficient to place holes in film containers through which the acid vapours may escape since they may infect neighbouring films which are in good condition. The acid may be reduced by aerating the film periodically, though rewinding film is a labour intensive process.
What is needed is an inert scavenger of the acid, and this has recently been provided by Kodak's Molecular Sieves. The sieves reduce the build up of acids in the film can preventing secondary reactions taking place (refer to the work of Tulsi Ram) The film can itself also plays an important role in film stability. Recent studies show that aluminium cans are superior to tin-plated metal cans. Tin-plated cans are subject to rusting by evolved acids and may themselves contribute to oxidative breakdown of films, especially for nitrate film.
Although peroxides in cellulose acetate film are considered to be stable below 160ºC, their decomposition may be catalysed by metal ions at ambient temperatures. Aluminium cans do not appear to facilitate such processes.
Plastic cans commercially produced are either polyethylene or polypropylene or copolymers of these two. These plastics contain inherently greater levels of peroxides than photographic films themselves. The peroxides in plastic cans are frequently less stable than those in photographic film and their decomposition may instigate degradation reactions in the films they are in contact with. The plastic can may absorb some of the acids emanating from the film it holds, but this does not necessarily offer any beneficial effects. More research into suitable film containers is still needed.
Control of environment will never be enough and break down of film is inevitable. It follows that along with appropriate environmental control, a test is needed to identify the end of the useful life of a film. Herein lies the crux of the problem: How do we define the useful life of a film? It has been proposed that if acidity levels build up so that pH reaches <4, then the film should be copied. So
How can we test for degradation?
How can we test for degradation?
From the moment a film is produced it begins to degrade slowly. During the time period prior to auto-catalysis there is a gradual build up of acid in a film. It is possible to identify the acidity threshold to auto-catalysis by means of an indicator which changes in colour, when the acidity threshold is crossed.
A number of such indicators are currently commercially available. For acetic acid these include IPI's A-D Strips and Dancan's Dancheck. For nitrate film: the Alizarin red-dye test is available. In this test a hole punch of film is placed inside a test-tube. Filter paper soaked in a solution of the Alizarin dye is then placed in the neck of the test-tube. The sample is then heated and the time for the red dye colour to be bleached is noted. A film is considered to be in good condition if little change in colour has taken place after one hour.
Generally, such colour indicators are not stable to light or require long exposure times (24 to 96 hours or, at the best, 1 hour) before a colour change is noticeable. Irrespective of these problems, it is envisaged that archivists may require more than one type of indicator.
- On accession of a film by an archive, an instant-response indicator is required to give immediate feedback on the condition of the film.
- When the film is shelved in the archive we require an indicator which responds to gradual build-up of acid within the film container. Here it must be appreciated that when a film is taken from the vault and the lid removed acids will escape and it will be necessary to replace the lid and leave for a further period of time when an indicator is put in the can to allow acids to build up again, in any event this will not reproduce the acidity levels which built up over years in the can. The indicator should be in constant contact with the film in the can in the archive and its change of colour should not be reversible.
- A very sensitive on-line sensor placed in the storage area capable of monitoring minute fluctuations in acidity during the course of a day would maintain optimum humidity and temperature corresponding to minimum degradation.
With this in mind indicator tests are being developed for acetate film at the Centre for Archival Polymeric Materials. One test uses an indicator dye which changes colour from red to yellow; here a hole-punch film sample does not need to be heated but left for 1-2 hours at room temperature. After this time dyed filter papers which show a linear colour change from their base of 1mm height or more require copying. This test method is rather labour intensive and time consuming so another indicator was developed by absorbing the dye onto a solid support.
A number of trials were carried out on films supplied by the North West Film Archive. A small quantity of the indicator was placed in the film can and examined periodically and the results are given in Table 4. The results provide a graded indication of the presence of various levels of acidity within the film can which apparently correlates with the tested pH of the film samples. A sample with pH<4 is yellow within 4 days of exposure whereas a sample of pH 5.74 remains red even after 14 days; between these two extremes a sample of pH 4.88 takes 14 days for 90% of its area to become yellow whereas a sample of pH 4.44 takes only seven days for the same amount of change. Therefore, the indicator is capable of operating over a long period when a film can is placed in a vault.
| Table 4 | Colour Change of Indicator Exposed to Acetate Films | ||||
|---|---|---|---|---|---|
| Sample | pH | 1 Day | 4 Days | 7 days | 14 Days |
| 3 | <4.00 | 50% Yellow | 100% Yellow | 100% Yellow | 100% Yellow |
| 28 | 4.44 | 10% Yellow | 50% Yellow | 90% Yellow | 100% Yellow |
| 21 | 4.88 | 100% Red | 100% Red | 5% Yellow | 90% Yellow |
| 18 | 5 | 100% Red | 50% Pink | 5% Yellow | 100% Pink |
| 5 | 5.74 | 100% Red | 100% Red | 100% Red | 100% Pink |
| 38 | n/k | 100% Red | 100% Red | 50% Pink | 50% Pink |
| 42 | 5.7 | 100% Pink | 100% Pink | 100% Pink | 100% Pink |
The test is relatively insensitive to nitrate film and the dye used is relatively light and pollutant insensitive: A sample place near a window in Manchester city centre had not changed colour after 6 months. It is possible to modify the response time of the indicator, and this has recently been achieved. On contact with severely degraded films the indicator changed colour irreversibly in less than one minute; so it may also be used as an instant response indicator.
In conclusion, though marked progress has been made towards preserving our audio-visual heritage there is still much to be done. Only by a continued effort on the part of archivists, scientists and manufacturers can we extend these horizons.
Questions from the Floor
Ian Gilmour:
It appears that water is the main factor in degrading magnetic tape, in particular polyester-urethane, and that this is typically a reaction where the hydrogen ions and hydroxyl ions separate to form an alcohol and cartezolic acid which then accelerates the reaction. In order to confirm this, a number tests were conducted in which tapes were artificially aged in both inert gases such as nitrogen and in oxygen. The results suggest that, in order of severity, hydrolysis occurs first and then, at higher temperatures, oxidation and then, finally, pyrolysis at well above room temperatures. This has led us to want to store tapes in much lower humidities. I was wondering if you have any comments to make.
Michele Edge:
It really depends upon the specific structure of the urethane elastomer. There can be great differences between samples from different sources. In some urethane elastomers hydrolysis will dominate the reactions. In our studies, we have found that we can quite easily see the different peroxide levels in different samples and we have observed that the peroxides are decomposing so there must he oxidative degradation taking place as well. We found that the moisture that was present was serving as a plasticizer to actually enhance contact between the urethane elastomer and the pigment particles. This then facilitated the oxidation.
Jacques Lemaire:
We have been working a lot on the competition of the hydrolysis and the oxidation and in most cases oxidation precedes hydrolysis. For example, in polyester urethane the oxidation process leads to alpha carbonyl rafae which are very sensitive to water. So oxidation precedes hydrolysis.
Michele Edge:
That is precisely what we found in our results - the initial mechanism was the oxidation.
Gerry Gibson:
The chart that you showed from the Image Permanence Institute demonstrated, for example, that at 80%RH and at 10°C the tape had a life expectancy of 70 years. At 20%RH and at 24°C it had a life expectancy of 60 years. It would seem to indicate, therefore, that temperature has a major impact. That has not been addressed here. Could you comment?
Michele Edge:
The principal mechanism of the breakdown is that, to form the acid, you need moisture. All chemical reactions are subject to temperature; the higher the temperature, the faster the process takes place. There is, therefore, an interchange between the temperature and the humidity levels. If you go to very low humidity, there is no agent present to cause the reaction. If there is humidity present, then the lower the temperature the better.
Gerry Gibson:
Can we conclude, therefore, that cold and dry is best?
Michele Edge:
It depends upon how dry and how cold, because of other physical property changes. It ceases to be an issue of chemistry and becomes more a problem of physical properties.
Henning Schou:
One thing that I do not fully understand is that, if you do not know exactly how much acetic acid, which is a catalyst, is present in a film, how can you predict the lift expectancy of the film?
Michele Edge:
This is also a problem. This is why we are working on an indicator. We want to be able to give an indication of how much acid can accumulate before the film is no longer viable. For nitrate film this is easy. You can directly correlate the nitrate acids given off with image fading. With acetate film, the acids create problems with copying, the emulsion becomes sticky and there are physical property changes. A simple correlation is not possible.
David Stebbings:
I wondered if anyone had studied the possibility of a microbiological activity causing the decay of these polymers.
Michele Edge:
One major area of polymer research is into biodegradation. The types of polymers that we use for tape and film supports are, in fact, reasonably resistant to bacteria. When breaking these polymers down for recycling, other materials are frequently added to try to accelerate the breakdown. What usually happens, however, is that the bacteria attack the introduced material and leave the polymer alone. So the support polymers seem to be proof against bacterial attack.
Tulsi Ram:
I want to confirm one point about cellulose nitrate films. We have traditionally thought that it is degrading faster than the acetate materials. The latest data from our tests of films in storage is showing that the pH of the nitrate films is noticeably higher. (i.e. it is less acid) when kept in the ideal storage conditions than the acetate. Accelerated aging tests are also indicating that the behaviour of nitrate film is better than acetate.
Michele Edge:
It depends on the parameter that you are measuring. If you are measuring the acidity levels then I agree. If, however, you examine what is given off by nitrate film when its temperature is first raised, then you will find nitrogen dioxide which fades the image.
Friedrich Engel: May I point out that not all binders of magnetic tape include polyurethanes. Some tape binders, particularly in tapes of German manufacture, are PVC based and these are more stable.
Michele Edge:
As Prof Lemaire will, I’m sure, confirm, PVCs are notoriously unstable, particularly with regard to oxidation and breakdown. It really depends upon the exact formulation. I have been talking about the work done on urethane elastomers and cannot, therefore, comment greatly about the PVC binders because we have not examined these in detail. The research has involved examining old tape samples which contain urethane elastomers.
Dietrich Schüller:
A comment about the Vinegar Syndrome and sound tapes. As a result of/he Second JTS in Berlin in 1987, we examined the tapes held in the Phonogrammarchiv of the Austrian Academy of Sciences and found a tape that smelt of vinegar. It was removed from the store and placed on my desk in an open box. I have now lost an interesting demonstration item because it does not smell of vinegar any more and it is perfectly playable. I guess that there is a trade off between ventilation and enclosures; between the vinegar syndrome and dust and atmospheric pollutants.
Michele Edge:
You are right; it is the practicality. On one hand it is enclosure and the vinegar syndrome and on the other good ventilation and dust. If you merely punch holes in the boxes and cans, you run the risk of infecting adjacent films or tapes.
Ian Gilmour:
The point raised by Dietrich Schüller is timely. The indicators that have been discussed measure the concentration of acetic vapours in the air. There has been a lot of debate recently about whether it is better to aerate cans or whether it is better to keep them .sealed so that a decaying film does not infect its neighbours. Could you advise on this please.
Michele Edge:
I cannot really comment on this dilemma. It is really for you to decide which course of action will he best for your collection. My work is aimed at finding out what is happening to enable you to make informed decisions.
Morten Jacobsen:
Dr Edge said that she would group indicators into two groups: long-term and short-term. The indicator that my company make, the M-Check, is placed by her in the long-term group. If I put one of my indicators in a can with a film that is only marginally smelling of vinegar, even to the nose at the start of a day when it is fresh, my indicator will show a change within a few days. If a similar test is carried out with a strongly acidic film, the indicator will change within six hours. This I would call a short-term indicator.
We must also look at how long the indicator will stay in the can. Will it last far a generation or two or three? The indicator must survive as long as it is necessary for it to turn yellow.
The question of ventilation of cans is interesting. There are libraries in the USA where they hold the lid of the can above the base by means of small pegs. Others punch holes in the base and sides. Both increase ventilation considerably. There are countries, however, where the fire regulations do not permit this.
Michele Edge:
The grouping of indicators into long-term and short-term is merely a guide as to how long it will take to change colour. There are several good indicators available, including yours and the one Joan was speaking about, that change colour in a day or in a week or so. What is needed is an indicator that will change colour very quickly, ideally with a graded colour change that permits an estimate of the degree of acid to be made, so that films can be tested as they come into the archive.
Henning Schou:
May I ask Joan a question. You said that you use non-ionic resins for these tests. As the chemists amongst the audience will know, when you measure the pH it is, in fact, the negative logarithm of the ionised hydrogen. How confident are you that a non-ionic resin will give a realistic result?
Joan Whitehead:
I am confident because what I have done is to change the pH range of the indicator that I am using by buffering it. Also I am not measuring the pH of the film but the acetic vapour in the can. The results of the tests that I am conducting cannot be expressed in terms of a pH anyway.
Henning Schou:
An acid is only as strong as its medium and when we measure pH we talk about water as neutral at 10.7 of hydrogen etc. If the water is not present and we use non-ionic resins, presumably without water, how reliable are the results of your work?
Joan Whitehead:
I will be able to answer that point in three or four months time. The work is still in progress but the signs are that the test is going to be very reliable.
Henning Schou:
Would it he true to say that, at the moment, these indicators are at an experimental stage?
Joan Whitehead:
Yes.
Terry Watson:
The lithium red test that you described showed a colour change after one or two hours. Does this tell us where on the graph of acetate film that particular film is? Has it already passed the point of no return?
Joan Whitehead:
What I found was that a one millimetre linear colour change on the filter paper corresponded with a pH of about 4.0 or 4.5. I cannot he more specific because the lest is carried out at room temperature. Variations in the ambient temperature cause variations in the results. Tests carried out in the higher temperatures of the summer months reacted much faster than tests carried out in the winter. I have carried out tests on over 100 films and I can confidently say that if the filter paper shows a one millimetre colour change then its pH is between 4.0 and 4.5.