The deterioration of polymers in audio-visual materials
THE DETERIORATION OF POLYMERS IN AUDIO-VISUAL MATERIALS
Dr Michele Edge, Manchester Metropolitan University, UK
Presented at 1990 JTS in Ottawa; printed in the Proceedings Pages 29-39.
ISBN 1 873902 02 6
Published by the Technical Co-ordinating Committee
IASA members may download a PDF version at no cost. If you are not a member, why not join IASA?
Introduction by Henning Schou:
The next speaker is Michele Edge, who obtained BSc and PhD degrees from Manchester Polytechnic (now Manchester Metropolitan University) and is currently a Lecturer in the Chemistry Department of the same institution. She has widespread experience in photographic image related materials, especially with regard to their deterioration and stabilization, with twenty research publications to her credit. Together with Professor Norman Allen and Dr Jewitt of the Polytechnic and Mr Velson Horrie of the Manchester Museum, she has been primarily responsible for establishing the Centre for Archival Polymeric Materials, whose concern is to provide a consultative service to the archival and conservation worlds regarding polymeric materials.
Table of contents
Introduction
In Manchester we are setting up a Centre that will provide a service to all archives and museums dealing primarily with polymer stability. I will start by summarizing the problems that we have to date: we once believed that we could just acquire and save audio-visual collections and amass data; but, once acquired, the collections truly become a nuisance because they have, as a major constituent, polymers. Now all polymers are unstable with time and therefore have a limited life span. So any materials that contain a polymer system will be unstable and will deteriorate, and that includes all current information storage material.
Why do polymers break down?
Polymer degradation is a feature both of the intrinsic chemical structure of the material itself and of environmental parameters. For a given material, a number of external and environmental factors can influence the rate of degradation. These include:
- the effects of oxygen,
- the effects of moisture in the atmosphere, leading to hydrolytic breakdown,
- heat, leading to thermal degradation,
- mechanical stress, leading to loss in tensile properties, for example,
- the effects of light,
- radiative breakdown,
- chemical breakdown due to atmospheric pollutants,
- biological breakdown - the growth of fungi, mould etc on the surface of polymer materials,
- ultrasonic breakdown.
The action of all those factors upon a particular polymer results in a number of property changes that can be divided into two groups, physical and chemical changes.
- Physical changes include a decrease in molecular weight and hence loss in tensile strength for the polymer system, loss of gloss, elongation, distortion, etc.
- Chemical changes are those that result in discoloration of the system, production of new groups within the polymer system itself.
The same changes happen in motion picture film, which is essentially composed of a polymeric support. It does not matter whether it is cellulose nitrate, cellulose triacetate, or polyester, and the same is true for magnetic media, and for emulsion containing the silver image and gelatine or a urethane binder and magnetic oxide. In all cases, the binders are polymeric and the support materials are polymeric.
I said earlier that the stability of a given polymer will be dependent upon both the innate structural features and external environmental parameters. The structural features of most polymers are:
- the main polymer unit itself, the form in which it’s present, its physical form, whether it’s present as a film, whether it’s present in bulk, or whether it’s present as a powder - all these influence stability;
- the presence of impurities within the system from manufacturing catalysts, for example metal trace ions that it has picked up from washing procedures;
- the presence of incorporated additives, such as plasticizers (which are generally added to polymers to render them flexible), pigments, antistatic agents and flame retardants.
What is a polymer?
A polymer is essentially made up from small organic molecules linked up into long chains. In some cases the structures are linear, others have pendant groups that project on either side of the main chain (the monomer unit).
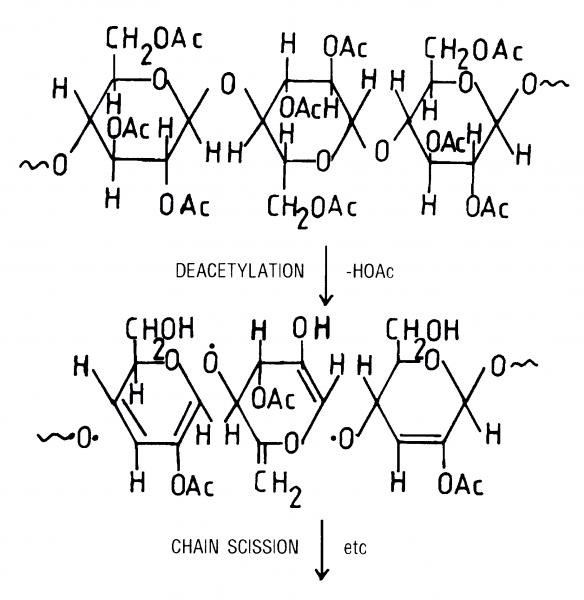
Figure 1
If we look at the chemical structure of a typical motion picture film support such as cellulose nitrate and cellulose triacetate (included in Figure 1), we see that basically it’s composed of a cellulose backbone made of small hydroglucose units linked together to form a long chain polymer; substituted in that main backbone are either nitrate groups (in the case of cellulose nitrate) or acetate groups (in the case of cellulose acetate and cellulose triacetate). So, our support material is a polymer which is a backbone with pendant groups.
A number of energy considerations have to be taken into account when looking at the stability of a particular structure. The first are the intramolecular forces - the bonds between each atom within the molecule. Basically, the stronger the chemical bond, the more stable the polymer will be and the more energy it takes to break that bond.
For example, a carbon-fluorine bond would be need 116kCal of energy per mole to break it; the carbon-hydrogen bond (present in cellulose acetate and cellulose nitrate) requires only 97kCal per mole; the carbon-carbon bond (also present in both support materials) requires only 83kCal per mole. The stability of the polymer also depends on the type of carbon-hydrogen bond present. If the carbon’s bonded to three hydrogen atoms, for example, it’s more stable than if the carbon’s doubly-bonded to another carbon: instead of just one bond, there are two.
If we look at intermolecular forces, we find that the many, many polymer chains within our plastic support will interact with each other and form weak links between the chains.
If you wrote a league table of bond strengths within polymer systems, then the bonds between the atoms that constitute the polymer chain take most energy to be broken, the intermolecular forces take a little less, and there’s essentially a cohesive energy between the chains. The cohesive energy in a rubber system, an elastomer which you can stretch, is pretty low compared to, say, a nylon or cellulose fibre where the cohesive forces will be much stronger. The presence of polar groups and hydrogen bonding provides the possibility of relatively strong links between each particular chain. So when you examine a polymer structure, you have to take into account whether all those forces or bond linkages can actually occur.
What happens when support materials break down?
Using the particular examples of the support materials cellulose acetate and cellulose nitrate, how do we actually utilize information about the structure and structural stability to study what’s occurring when those two support materials breakdown?
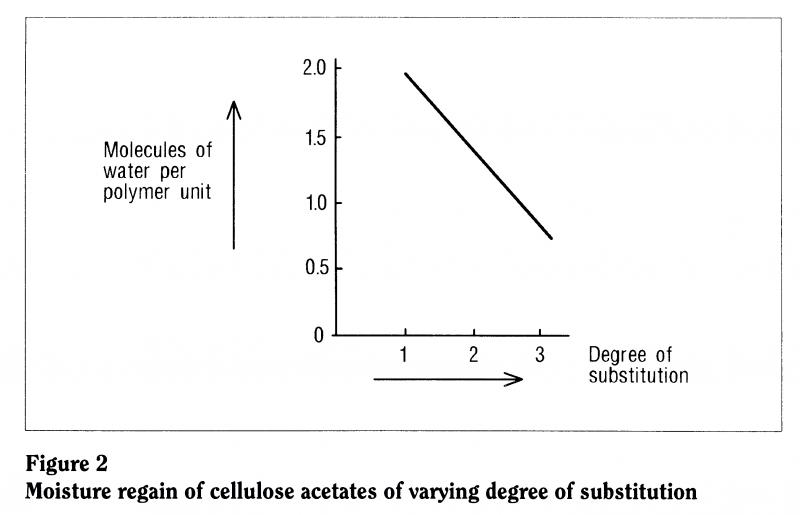
A particularly important feature of cellulose acetate and triacetate and, to a lesser degree, cellulose nitrate base is the extent to which the polymer can interact with the environment and take up moisture. Studies have shown that, as you increase the degree of substitution (as you go from monoacetate to diacetate to triacetate, see Figure 1), then you lower the moisture uptake of the polymer. The higher the degree of substitution, the less moisture it takes up. So conversely, if the polymer is degrading C giving off acetic acid, losing acetate groups, lowering the degree of substitution Cit will start to take up more moisture (Figure 2).
Another feature is the polymer’s solubility. The specific structural composition of the molecule will influence the way it interacts with solvents and hence will influence which solvent systems it can actually dissolve in. For example, at higher degrees of substitution, cellulose acetate is soluble in systems like methyline chloride and methanol which is actually used to cast film base and, as at lower degrees of substitution, it becomes increasingly soluble in more polar solvents until, at degrees of substitution of only 0.6 acetate groups per anhydroglucose monomer unit, the system should be soluble in water. You can actually utilize that information to study what is happening in your film material.
At Manchester Polytechnic, we took a series of films - naturally aged cellulose acetate films of unknown history, and a number of negative and positive films that we knew the dates of - and looked at them in several ways. Our initial observations of what happens to triacetate film when it degrades suggest that, when we smell acetic acid, this has come from acetate groups - the pendant groups that are substituted on the main chain. If we’re losing those groups, we’re lowering the degree of substitution and, therefore, we should see an increase in the uptake of moisture. We should also see, as we form acetic acid, a lowering in what we call the pH of the system. [If something has a high pH, say 13 or 14, it’s essentially alkaline/basic. If it has a very low pH, it’s tending towards being acidic.]
We can also utilize the information on solubility in a given solvent by measuring what we call percentage insoluble polymer. If we’re losing acetate groups (lowering the degree of substitution) then, if we could dissolve our original triacetate material in something like methyline chloride and methanol, it should become progressively insoluble in that particular solvent. So we can get a measure of percentage insoluble polymer.
We can also measure the viscosity of the system. We’re losing the pendant groups from our long chain to form acetic acid and the fact that we see a loss in tensile strength when we project films (the film snaps in the projector), suggests that the polymer unit itself, the main backbone, is also breaking down to smaller fragments. For a long chain, you can imagine that all the chains are entangled and it’s a relatively viscous solution. As the chain breaks into smaller fragments, it flows more freely. We should be able to follow that by measuring how viscous the solution is.
When we look through films taken from an archive, we find that some films have lost their image and also smell of acetic acid and that, the more degraded the film material, the higher percentage insoluble polymer it has, the lower its pH, the higher the moisture regain, and the lower the viscosity number. This seems consistent with the idea that the polymer is actually losing acetate groups to form acetic acid, and its main chain backbone is breaking down to smaller fragments.
The effect of age
The effect of age It is difficult to find any age-related changes; some of the older films are in much better condition than some of the newer ones. In an archival situation, you do not know the previous history of the films that come into the archive. So we decided to take the same set of readings for films that had actually been produced on site at an archive and stored under archival conditions (45-50% RH and ambient temperatures). There we did see a trend: the older the material, the lower the viscosity, the higher the moisture regain, the lower the pH, and the higher the percentage insoluble polymer. So the environment does have an effect, and the age of the polymer is relevant. But, remember, archivists often don’t know where films have come from; they could have been exposed to any environment, giving a whole range of deterioration characteristics.
After looking at a series of naturally aged film stocks, we used all the same testing procedures and simulated degradation to see if we could, perhaps, predict how long a film would last under a given set of conditions. We took the same parameters and looked at what happens to film in dry conditions, in conditions of 100% RH, and in conditions of one molar acetic acid, all at a series of three different temperatures. Taking moisture regain as an example: as the temperature is increased, the onset of moisture regain is more rapid. So, increasing the temperature speeds up the rate of degradation.
That’s not new, but the effect of moisture in the environment is particularly significant, and is much more marked than the effect of temperature. At 90°C, in molar acetic acid conditions, the moisture regain C which corresponds with the loss of acetate groups and the formation of acetic acid is almost catalytic. There’s only a very, very small induction period before the acetate groups start to be lost.
What’s happening to the polymer is a complex interplay between the acetylation and the breakdown of the main chain: chain degradation is occurring and also what we call hydrolytic de-esterification C in other words, the loss of acetate groups is enhanced by the presence of moisture.
The effect of plasticizers
But that’s not the whole story. Photographic film base incorporates a plasticizer at around 15% by weight of the film base and that’s, typically, a phthalate ester combination with triphenyl phosphate. In many severely degraded films, white crystalline deposits can be seen on the surface of the film material itself. We ran chemical tests and discovered that the crystalline deposit was plasticizer material. What influence might the plasticizer have? It must be playing some part, whether it be active or merely facile, because it’s changing - coming out of the support material itself.
Most phthalate testers (which include dimethyl, diethyl and dibutyl) have lots of CH2 linkages that are particularly unstable and susceptible to breakdown by the presence of oxygen. In going from dimethyl to diethyl to dibutyl, the amount of oxygen taken up increases. We believe that, under the influence of oxygen, those systems then break down to produce what we call peroxides and acid. Triphenyl phosphate doesn’t possess any of those units and so apparently doesn’t absorb any oxygen and doesn’t produce any acid or peroxide.
When polymers break down, when they interact with oxygen, they produce peroxides, which are notoriously unstable, and break down to form radicals. Now a chemical bond is composed of two electrons and is relatively stable, but when a chemical bond is broken to produce radicals, the radicals have only one electron - and electrons, like people, don’t like being alone. They’d rather be paired up. So any radical on its own will seek another electron; if the electron is paired up in another bond, it will grab hold of it and break the bond. This will produce more radicals, and the whole thing takes off and produces more and more radicals. The result is more and more broken chemical bonds. The presence of peroxides in a polymer system is, therefore, particularly important.
We decided to artificially age polymer systems - movie film - with and without plasticizer material, and look at the viscosity retention as one parameter relating to that breakdown. In the presence of plasticizer, the film seemed be in much better condition and retained its viscosity to a much higher degree than when no plasticizer was present. We wondered if the plasticizers somehow contributed to the stability of the polymer itself. The plasticizer was obviously being lost from the polymer, so we looked at percentage plasticizer loss in a given time, at a given temperature, under conditions of high relative humidity and acetic acid conditions. In the presence of acetic acid, a far greater proportion of plasticizer was lost from the film.
The plasticizer is designed so that its chemical structure is specifically compatible with the original base material. We believe that, as the base material changes its chemical composition (loses acetate groups, the chain breaks down, etc), the plasticizer is no longer compatible with that system and is pushed to the surface, where it crystallizes out. So, plasticizers don’t contribute to the instability of the material, rather they are lost because the material itself is breaking down.
The effect of the film can
But that’s still not the whole story. The film is not just sitting on the shelf tightly wound into a reel, it’s actually in a film can. Is the can itself contributing to the instability of the material?
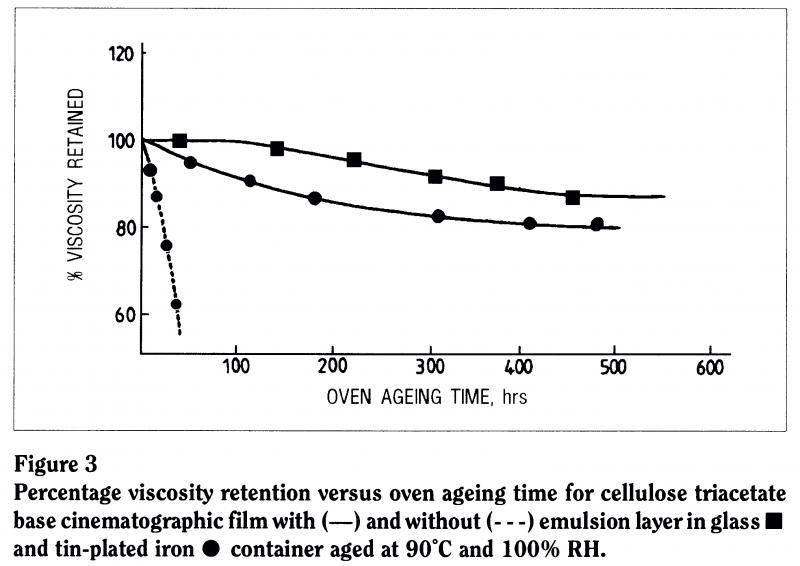
Metal ions are notorious in the breakdown of polymers. The chemical literature tells us that metal ions contribute to the instability of almost every known polymer system: they act as catalysts for their breakdown. We looked at what happens to photographic film, motion picture film and triacetate base in contact with a can. In one case, we removed the emulsion from the film, in another we left it on, and in a third case we left the emulsion on and aged the film in contact with glass rather than with a metal storage can (Figure 3). The support without emulsion tends to degrade first, the film with emulsion in contact with the can takes a little longer, but the film in a glass container is much more stable.
We also looked at a number of naturally aged film materials taken from film archives, at new materials, at film that was exhibiting acetic acid odour, at films that had plasticizer deposits, and at films that had lost the image altogether. The analysis was done by Kodak in England who found that, the more degraded the film was, the greater its iron content. In other words, the film material, as it degrades, seems to be taking up iron. Now it’s difficult to tell from those two studies whether or not this is a facile feature of the degradation. It maybe that, because the film takes up more moisture it also takes up more metal ions along with that; so the iron isn’t actually contributing to the degradation, it’s just being taken up as the film degrades.
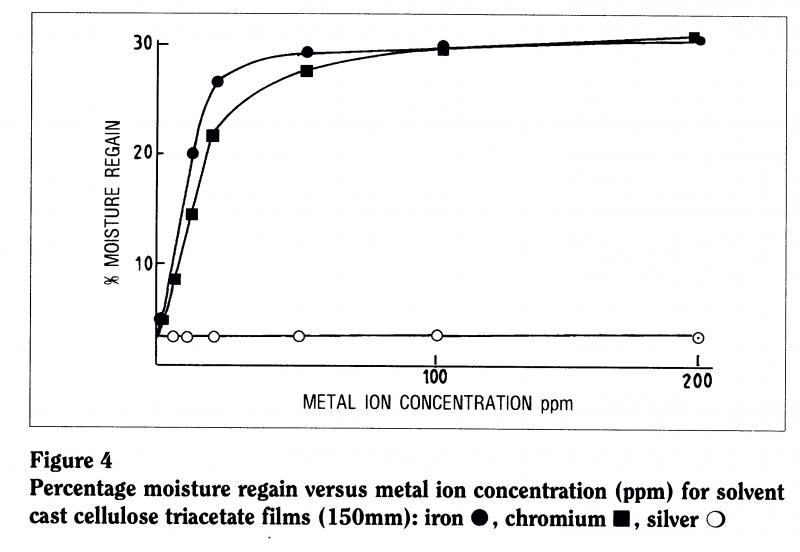
To check that, we actively incorporated different concentrations of metal ions within a number of different films (Figure 4). When we put one part per million of ions into the material, there wasn’t much change in the viscosity the film wasn’t degrading very much. But, as we increased the concentration to ten parts per million, there was significant loss of viscosity Csignificant degradation. In other words, iron contributes to the instability of the material. So it’s likely that the film can is contributing to the instability of the material.
And with magnetic track media, because the magnetic track is an iron oxide or iron itself, then that must also be contributing to the instability. We took from the archives a cross section of materials that we knew were exhibiting degradation and we looked at the percentages of degraded film materials exhibiting acetic acid odour (or vinegar syndrome). We found that 78% of the degraded materials were magnetic track materials, compared with only 22% that had no magnetic track and so had no iron present in the base material.
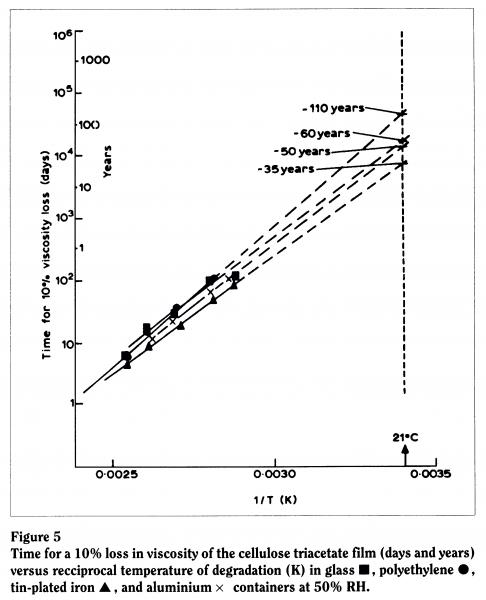
Because we realized that the effect of the film can was particularly important, we used Arrhenius testing methods to ascertain the time taken for a given loss in a physical property of the material. For example, we plotted the time taken for a 10% loss in viscosity against temperature in various storage media. At 50% relative humidity (the humidity level found in many archival storage facilities), we found that film was most stable in glass, then plastic, then aluminium, and least stable in contact with a tin-plated can. We predicted that the lifetime of the material in contact with a typical tin-plated can in an archive would be around 35 years (Figure 5). Allowing for experimental error and any error in our plotting (given that we’re plotting against a log scale, our line could be out by "10 years), this corresponds reasonably well with what we actually find because, if you recall, triacetate was introduced in the early fifties and we’re seeing degradation now.
The effect of the emulsion
Because film without emulsion breaks down more rapidly, another question arises: what part is played by the emulsion itself - by the presence of gelatine? It seems that the gelatine is actually acting to stabilize the material, probably mainly because gelatine is itself a polymer system made up of amino acids and linked into long chains, into a protein-type material. When the degradation products (the acetic acid) from the base hit the emulsion, the emulsion serves as a mop for the acid. When it takes up the acid, the emulsion itself is broken up and is lost: you can then wipe off the silver image and the emulsion becomes sticky. Because film is wound tightly into a reel and is, therefore, a sandwich of support material and emulsion, we believe that the emulsion also somehow acts as a diffusion barrier to, perhaps, the ingress of oxygen.
Also, negative film has a thicker layer of emulsion than positive film, so we took a cross section of degraded stocks in an archive and compared percentage incidences of positive and negative films exhibiting vinegar syndrome. We found that positive films formed around 59% of degraded material compared to negative films only 41%. The emulsion layer itself is beneficial.
The effect of an enclosed environment
So far we had looked at all the constituents and at the fact that the film is in a can; but what about the fact that, because the film is in a can, it is in a smaller environment than if it was just on a shelf? We ran infra-red spectra, which give a trace corresponding to particular groups in the molecule.
We looked at some cinematograph films, and the first thing we noticed was that the moisture content was changing significantly: it was taking up moisture. We already knew this, but the loss of acetate groups did not appear to be particularly great. Not many acetate groups are actually being lost in a film ageing from new to loss of image.
We were lucky enough to get hold of a triacetate sculpture that had stood in an open environment for 20 or 30 years. It was exhibiting the formation of acetic acid - the vinegar syndrome - but, in this case, the degradation patterns were quite different. It didn’t appear that the sculpture was taking up moisture but that, in the place of the acetate groups on the polymer backbone, we were actually substituting hydroxyl groups in some way, and there was a massive loss of acetic acid. So it seems that in an open environment we are promoting loss of acid, and in a closed environment we are setting up some kind of equilibrium that prevents further loss of acid.
We decided to check that by artificial ageing techniques and found that, in an open environment, the onset of moisture regain (which corresponds to loss of acetate groups) is much greater. The onset is much more rapid than in an enclosed environment, where the loss of acetate groups will be suppressed. Unfortunately, however, we seem to have this Catch 22 situation whereby, although we’re subduing loss of acetate groups and de-esterification and forming less acetic acid than in an open environment, the polymer chain is breaking up instead. In an open environment we see very little decrease in viscosity or loss of tensile properties compared to an enclosed environment where it appears that, because we’re actually containing the acid impurities within the film can, they can serve to break down the main polymer chain which loses its viscosity and will also lose its tensile properties.
Using the information
Now we know more or less what happens when storing film.
- Storing it in contact with iron isn’t good.
- The emulsion is beneficial, but it will be lost as a consequence of base degradation.
- Humidity is more important than temperature in controlling film stability.
Having gained this information, what do we do about all these things? One option is to utilize this information to help decide when to copy the material that is degrading: we can use the chemical information about how degraded the film is to produce stability tests so that, at least, we can prioritize copying. Alternatively, we can stabilize the material itself, though to stabilize an individual polymer requires a recognition of the fact that it is contributing to its own instability.
Bearing in mind the major features that contribute to the instability of the film, if we are to stabilize the material, we must introduce something that can block the effects of all those particular factors: oxygen, the presence of any metal ions, the presence of elevated temperatures and moisture - all of which help to produce radicals or acetic acid that break the system down.
The particular combination that we have looked at is a tris-stabilizer, which is a combination of:
- a hindered piperidine stabilizer, Tinuvin 770, which acts as a mop for radicals and also, because it is basic, as a mop for any acid products,
- a metal de-activator, Irganox MD 1024, which complexes the metal ions so that they cannot accelerate the break down of peroxides to radical species,
- an anti-oxidant to counteract the effects of any radicals or any oxygen.
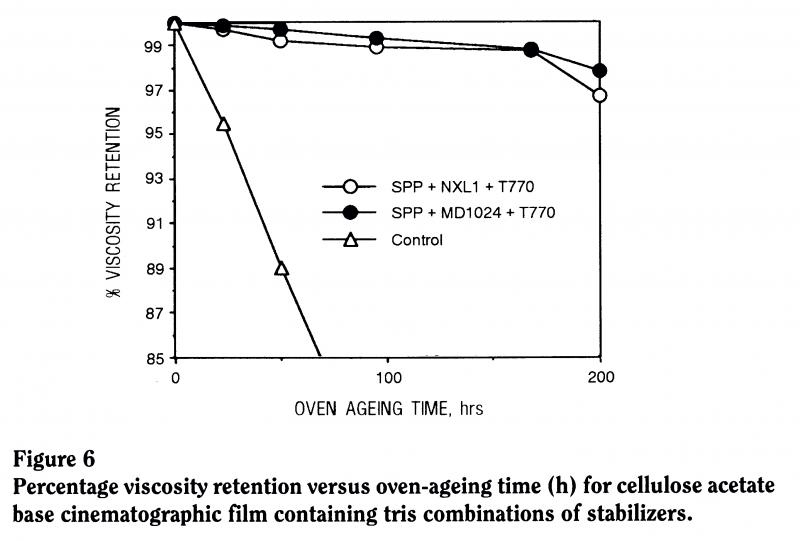
Comparing the property retention, the viscosity retention, of a controlled material with no stabilizer and a material with this tris-stabilizer system, you can see that the retention of properties is much superior with the stabilizer present (Figure 6).
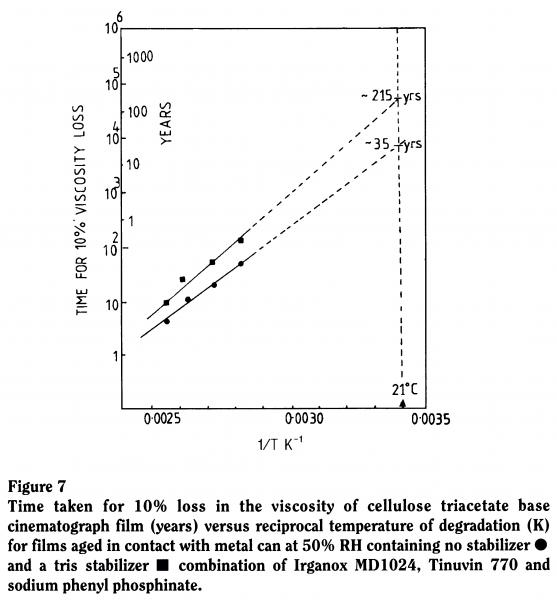
Our Arrhenius testing predicted that in a metal can, stored at 50% RH, with no stabilizer the film would last 35 years. We believe that the stabilizer will give a six-fold increase in life to around 215 years (Figure 7).
Question
Question:
Will having painted or enameled cans, instead of bare metal cans, retard the problems with iron?
Michele Edge:
The problem is that, when you coat a metal can, you can’t exclude trace metal ions from the surface, and as little as 10 parts per million actually promotes degradation. The other factor is that, if you coat a can, you’re introducing another plastic material that can itself degrade: over the long term, we have problems. Think of library books with sticky-back plastic covers; if you leave them in the drawer for too long, you’ll find that they stick together. That’s basically because you get inter-migration of additives between the systems and, because the additives in one system aren’t compatible with those in the other, breakdown is accelerated.